Which of the Following Elements Has Two Valence Electrons
If a neutral Magnesium atom loses two electrons what will its overall charge be. There are over 100 different borate minerals but the most common are.

Valence Electrons Characteristics And Determination Of Valence Electrons
Most elements that are essential in chemistry require eight electrons in each shell to be stable.
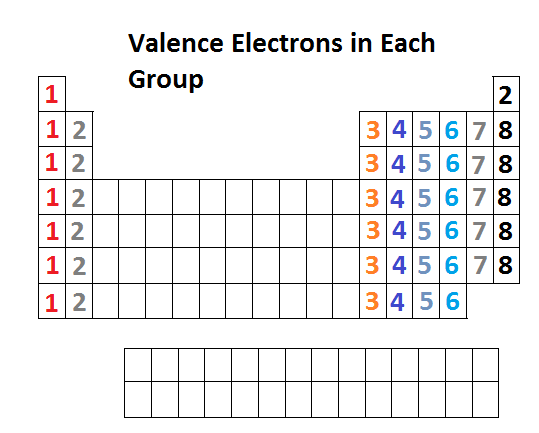
. In the p block the number of valence electrons is equal to the group number minus ten. Group 13 has three valence electrons Group 14 has four up through Group 18 with eight. It is not possible to determine the valence electron without electron configuration.
There is an article published on this site. Two fluorine atoms for example can form a stable F 2 molecule in which each atom has an octet of. Because each oxygen atom needs six nonbonding electrons to satisfy its octet it takes 18 nonbonding electrons to satisfy the three oxygen atoms.
Boron was known to the ancient Egyptians but only in the mineral borax. C has electronic configuration 2 7 and has seven valence electrons. Click here to check your answer to Practice Problem 1.
How many valence electrons are in each shell. A39 B18 C19 D20 B An atom that has gained one or more electrons has an overall negativecharge. B has electronic configuration 2 4 and has four valence electrons so belongs to group 14 and period 2.
9 Protons 8 Neutrons 17F 9. Na Mg and Al are the elements having one two and three valence electrons. The valence electron has to be determined by following a few steps.
The following steps are adopted for writing the Lewis dot structures or Lewis structures. 17F 9-1 F-1 9 Protons 8 Neutrons. In protonated methanol oxygen has 6 valence electrons 2 electrons in lone pairs and 6 bonding electrons therefore the formal charge 6-2126 1.
Counting the total number of valence electrons of one nitrogen atom two oxygen atoms and the additional one negative charge equal to one electron. It is even easier to see this if we use a short-hand description of the electronic configuration of each atom in which the electrons that make up part of a Noble Gas. The electron configuration is one of them.
Significant concentrations of boron occur on the Earth in compounds known as the borate minerals. A-2 B1 C2 C D-1. Knowing the electron configuration in the right way it is very easy to determine the valence electrons of all the elements.
In the s block Group 1 elements have one valence electron while Group 2 elements have two valence electrons. Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure. It is not possible to determine the valence electron without electron configuration.
Atoms of group 2 elements have just 2 electrons in the highest energy level also known as the valence shell of electrons. A Si b Mn c Sb d Pb. Answer the following questions associated with these elements giving reason in each case.
The electron configuration is one of them. The valence electron has to be determined by following a few steps. All of them contain two shells and so belong to second period.
Going down group 2 from top to bottom the elements display the following general trends. Borax kernite ulexite etc. 17F 9-1 F-1 Fluoride Ion 9 Protons 8.
Determine the number of valence electrons in neutral atoms of the following elements. A atomic number and therefore charge on the nucleus nuclear or core charge increases b number of valence electrons increases c atomic radius decreases d first ionisation energy increases f electronegativity increases excluding neon g elements on the left are metals elements on. Each oxygen atom in the ClO 3-ion already has two electrons the electrons in the Cl-O covalent bond.
The two elements following argon in the periodic table are potassium with a single 4s electron and calcium with two 4s electrons. The group has also gained two collective names earth metals and triels. The elements 4 Be 12 Mg and 20 Ca each having two valence electrons in their valence shells are in periods 2 3 and 4 respectively of the Modern Periodic Table.
Therefore the first regular transition series begins at this point with the element scandium which has the electron configuration Ar4 s 2 3 d 1. The chemical symbol for Boron is B. A Lewis structure with placeholder central atom is shown below.
Which orbital has the highest value of n. Because of the presence of the 4 s electrons the 3 d orbitals are less shielded than the 4 p orbitals. N 2s 2 2p 3 2O 2s 2 2p 4 1 negative charge 5 26 118e Step 2.
There is an article published on this site detailing the electron. The nonbonding valence electrons are now used to satisfy the octets of the atoms in the molecule. The following general trends are observed as you go across period 2 from left to right.
Knowing the electron configuration in the right way it is very easy to determine the valence electrons of all the elements. How many electrons are in an ion of K 1. The eight valence electrons a full outer s and p sublevel give the noble gases their.
However some atoms can be stable with eight electrons even though their valence electrons are in the 3n shell which can hold up to 18 electrons so it depends on the element. Atoms can combine to achieve an octet of valence electrons by sharing electrons. The latter name is derived from the Latin prefix tri-three and refers to the three valence electrons that all of these elements without exception have in their valence shells.
Total Number of valence electrons is. The metalloid element was not known in its pure form. 17F 9 9 Protons 8 Neutrons.

Finding The Number Of Valence Electrons For An Element Youtube

Valence Electrons Energy Levels Of Elements How Many Electrons Does Each Element Have Video Lesson Transcript Study Com

Valence Electrons Ck 12 Foundation
What Are Valence Electrons And How To Find Them Where Are They Located

Finding The Number Of Valence Electrons For An Element Youtube


No comments for "Which of the Following Elements Has Two Valence Electrons"
Post a Comment